According to the hardness table created by German scientist Friedrich Mohs in 1811, the softest metals are considered to be the alkali metals. The top five softest metals include: lithium – 0.6; sodium – 0.5; potassium – 0.4; rubidium – 0.3; and finally, the record holder for softness with a hardness rating of only 0.2 according to Mohs is cesium.
Mercury – The Softest Metal in the World
One of the most interesting chemical elements is mercury. This metal is constantly in a liquid state, which is why it is considered the softest metal in the world. To learn why mercury is liquid and other interesting facts about mercury, read here.
Mercury is widely used in more than 1000 areas – for example, in instruments, agriculture, mining, chemical industry, and more.
If we talk about metals that are typically found in a solid state, the top three leaders are potassium, rubidium, and cesium.
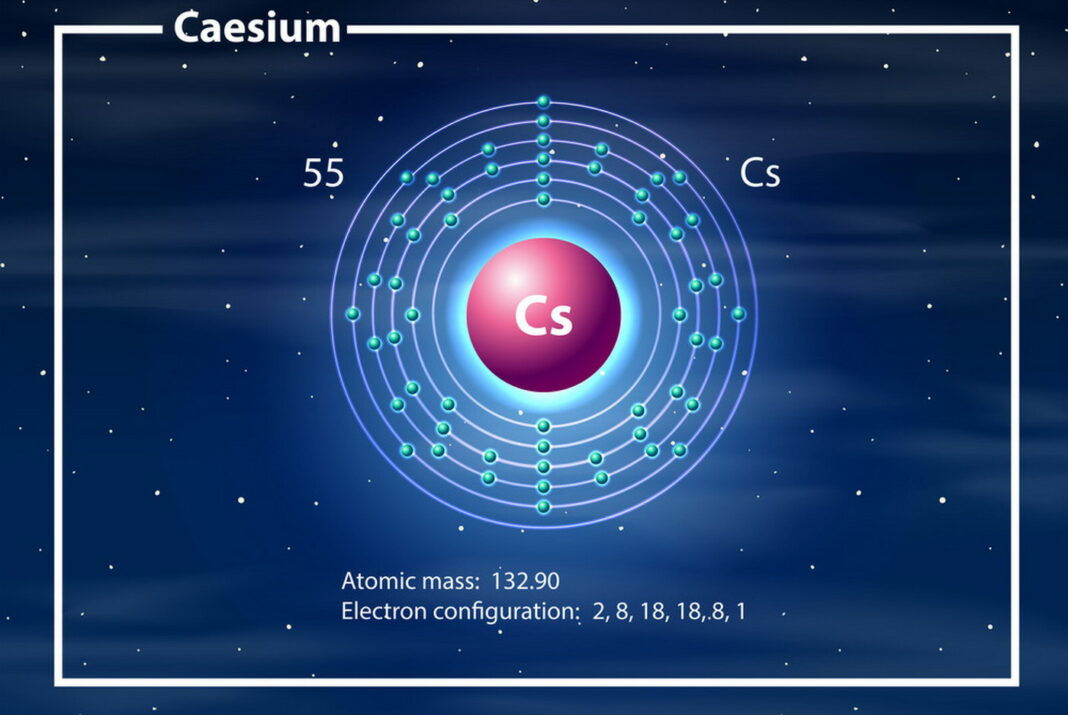
Cesium
In fact, this metal is so soft that it can be cut with a butter knife. Moreover, cesium melts at a relatively low temperature of -28.4 degrees Celsius (-83.12 degrees Fahrenheit).
This means that it will melt as soon as it comes into contact with a person’s hand, as the temperature of a healthy individual’s hand is 36.6 degrees Celsius.
It is important to know that touching this metal with unprotected skin is not advisable, as it is radioactive and can cause serious burns to the skin, so it should be handled with great caution.
Discovered by the German chemist Robert Bunsen in 1860, cesium has an atomic number of 55. The name of the element comes from the Latin word “caesius,” which means sky-blue. However, this does not reflect the color of the metal, which in its pure form is pale gold.
Cesium Carbonate
Gold, silver, copper, aluminum, and other ductile metals are also relatively soft, although much harder than cesium.
General Characteristics of Cesium:
- Type: Element (Minerals/Ores).
- Mineral Classification: Alkali.
- Chemical Symbol: Cs.
- Crystal lattice system: cubic.
- Color: Usually silver-yellow, resembling gold.
- Luster: greasy.
- Fracture: uneven.
- Isotopes: from 114 to 145 out of 31.
- Cesium 137 is the most dangerous radioactive isotope.
- Explodes upon contact with water.
Where is Cesium Used?
This element is used as a catalyst in chemical reactions. Since it easily ionizes with light, metallic cesium is used in photoelectric cells and infrared detectors.
Cesium compounds are also used in specialized alkaline batteries designed to operate in sub-zero temperatures. Cesium carbonate is used in the production of special glass and glassware.
The most accurate clocks in the world – “atomic clocks” – measure time based on the very precise vibration of electrons in the outer shell of a cesium atom. These clocks only lose 5 seconds every 300 years!
Cesium-137 is radioactive and can be used for radiation therapy to treat certain types of cancer and sterilize medical preparations.
Engineers for space travel have found that burning cesium in space becomes a very efficient form of fuel. It has been found to be 140 times more efficient than any other fuel.
Only a few thousand kilograms of cesium are used annually. Several minerals contain significant amounts of cesium, including mica, beryl, feldspar, petalite, and pollucite, from which it is usually extracted.
